Introduction:
Borax is a compound made up of an elementary substance known as boron that has been combined with oxygen and soda.
This article explains the borax formula, also known as sodium borate formula, sodium tetraborate formula, or disodium tetraborate formula, as well as its applications. A soft, colourless compound of Boron, borax is a substance that can be dissolved in water. The most common forms of borax are anhydrous and decahydrate salts, with pentahydrate salts being used occasionally.
Borax Chemical Formula
This form of borax contains four boron atoms and seventeen oxygen atoms, two sodium atoms, and twenty hydrogen atoms, making it the most common form of the compound. The following is the chemical components of borax:

Natural Sources of Borax
- Borax is found naturally in evaporite deposits, which are formed as a result of the recurrent evaporation of seasonal lakes.
- Naturally occurring borax is purified through the process of recrystallization.
- Borax can also be synthesised from various boron compounds.
Borax Reaction
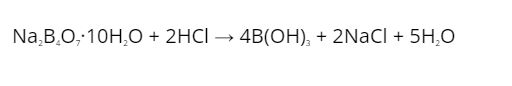
Borax is primarily used in the manufacture of laundry and other household cleaning products. It is also used to prepare boric acid, which is made by reacting with hydrochloric acid, or HCl, in a reaction vessel. Wilhelm Homberg was the first person to employ this method. Borax is also used in the food, chemical, and pharmaceutical industries, among other things.
When borax is combined with hydrochloric acid, boric acid is formed. The following is how people reacted:

Each of the sugar residues in the polymer has two hydroxyl groups positioned in the cis-form, which results in the Borax Reaction taking place. This results in an interesting and valuable reaction with dissociated borate ions, which is characteristic of polymers containing dissociated borate ions.
Uses of Borax
- As a Cleaning Agent – The various properties of borax contribute to the enhancement of its cleaning abilities. During the cleaning process, it converts water into hydrogen peroxide. Because it is highly basic, it contributes to the basicity of hot water, which increases the effectiveness of bleach or other cleaners.
- As an insecticide, borax works by interfering with the metabolic processes of many organisms. Because of this property, borax is a more effective disinfectant.
- It is used in the diagnosis of diabetes mellitus in humans.
- It is used as a water softening agent, among other things.
- A flux composed of a mixture of borax and ammonium chloride is used for welding iron and steel together.
Chemical properties of Borax –
- Because of its reaction with acids, borax can be easily converted into boric acids, which are extremely useful compounds. The following is an example of a reaction:
A yellow-green flame is produced by its combustion
- It is very soluble in ethylene glycol, and it is only slightly soluble in acetone.
- It reacts with sodium hydroxide to produce an acidic yellow-green flame.
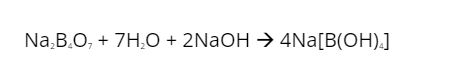
Conclusion:
For a long time, we’ve been cleaning with borax to keep our house clean. It was discovered for the first time in dry lake beds in Tibet, where it remained the only source until 1776, after which it was discovered in Italy, which became the primary source until the 1860s. In 1889, the famous 20-mule team borax company was established in Death Valley, California, United States. For a long time, it was the dominant player on the borax market.
Borax is a naturally occurring mineral that is also a salt of boric acid. Additionally, sodium borate, sodium tetraborate, and disodium tetraborate are all names for this compound. It is a boron compound that is important. Borax refers to a group of minerals that are closely related but differ in their crystal water content, such as the decahydrate, pentahydrate, and octahydrate salts, all of which are also known as borax. Even in its anhydrous form, borax is referred to as a substance.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






