The 16th group elements include sulphur (S), selenium (Se), tellurium (Te) and polonium (Po). This is often referred to as a group of chalcogens (due to its ore-forming nature). One of the most abundant of all the elements on earth is oxygen. Oxygen and sulphur are non-metals, selenium and tellurium are metalloids and polonium is a radioactive metal. The chemically uncharacterised synthetic element, livermorium (Lv), is predicted to be a chalcogen also. The metallic character, melting point, boiling point, density, ionic radius, and atomic radius increase as you go down the group from oxygen to polonium. On the other hand, ionisation energy reduces as we go down the table.
Oxygen Family or Chalcogens
The elements of group 16 include oxygen (O), sulphur (S), selenium(Se), tellurium (Te) and polonium (Po), having general electronic configuration ns2np4, are referred to as the oxygen family. These elements are also collectively referred to as chalcogens. Polonium is a radioactive element.
General Properties of the Oxygen Family
Atomic and ionic radii: Because of an increase within the number of shells, atomic and ionic radii increase from top to bottom within the group.
Ionisation enthalpy: Because of the rise in the size of the atoms, the ionisation
enthalpy decreases down the group. IE1 of group 16 elements are less than the
IE1 of group 15. This is often because group 15 elements have extra stability due
to half-filled p-orbitals.
Electron gain enthalpy: Because of the compact nature of oxygen, it’s less electron
gain enthalpy than that of sulphur. For the elements, which come after sulphur, the electron gain enthalpy decreases down the group.
Electronegativity: The electronegativity decreases down the group. this suggests
that the metallic character of the elements in this group increases when moving downward the group from oxygen to polonium.
Melting and boiling points: The melting and boiling points increase with a rise
in atomic number down the group.
Oxidation states: In group 16 elements, the outcomes of the oxidation state show ‒2, +2, +4, +6. In a ‒2 oxidation state, the stability decreases down the group because of a rise in atomic size and a decrease in electronegativity. O represents only ‒2 oxidation states except when it is amalgamated with the most electronegative F, with which it shows positive oxidation states. S represents + 6 only with O and F.
Anomalous Behaviour of Oxygen
Oxygen establishes strong hydrogen bonding in H2O, which is not found in H2S. Also, the
maximum covalency of oxygen is four, whereas within the case of the other elements of the group, the valence shells are often expanded and covalency exceeds four.
Reasons for the inconsistent behaviour of oxygen are:
• Small size and high electronegativity
• Absence of d-orbitals
Reactivity towards hydrogen: In group 16, all the elements form hydrides of the
category H2E (E = S, Se, Te, Po).
This is because of the increment in the length of the H-E bond when moving down the group, Therefore, the bond dissociation enthalpy eventually decreases when moving down the group.
Acidic nature: Acidic character of group 16 elements increases down the group due to the decreasing bond dissociation enthalpy.
H2O <H2S <H2Se < H2Te
Reducing character: Due to decreasing bond dissociation enthalpy, reducing character will also decrease when moving down the group.
H2O <H2S < H2Se < H2Te < H2Po
Oxygen (O)
In group 16, oxygen is the first element having an electronic configuration of 1s2 2s2 2p4 in the ground state. Oxygen has two allotropes: dioxygen (O2) and trioxygen or ozone (O3).
Dioxygen (O2)
Oxygen usually exists within the form of dioxygen.
Preparation:
Dioxygen is ready within the laboratory through thermal decompositions of oxygen-rich
compounds like KClO3.
Properties:
(i) Oxygen is colourless, odourless and is a highly reactive, tasteless gas.
(ii) Due to the presence of pπ‒ pπ bonding, O2 is a discontinued molecule and intermolecular forces are fragile Van der Waals forces, thus, O2 is a gas.
(iii) Dioxygen combines with metals and non-metals to make binary compounds
called oxides.
Examples are: 2Ca + O2 → 2CaO
P4 + 5O2→ P4O10
Uses:
(i) Dioxygen is utilised in making steel.
(ii) Dioxygen is additionally used for sewage treatment, river revival and paper pulp
bleaching.
(iii) it’s used as an oxidiser in underwater diving and in space shuttles.
Oxides
Oxygen combines with the bulk of the elements of the periodic table to form
oxides (O2-).
There are three kinds of oxides:
(i) Acidic oxides: Oxides of non-metals are generally acidic in nature.
For example, SO2 combines with water to offer H2SO3, an acid.
SO2 + H2O → H2SO3
(ii) Basic oxides: Metallic oxides are usually basic in nature. Basic oxides when mixed in water offer a basic solution. For instance, CaO combines with water to offer Ca(OH)2, a base.
Cao + H2O → Ca(OH)2
(iii) Amphoteric oxides: Some metallic oxides have dual characteristics of both acidic
as well as basic oxides. Such oxides are also known as amphoteric oxides. For instance,
Al2O3 reacts with acids also as alkalies
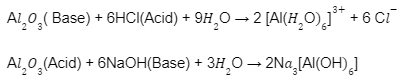
Conclusion
Also called chalcogens, group 16 (VIa) of the periodic classification consists of oxygen (O), sulphur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv). Tellurium was assigned its place by 1865, and polonium was discovered in 1898. As this group of elements has six valence electrons, they’re able to gain two electrons to point out a -2 oxidation number, and lose +2 oxidation number. Aside from oxygen, the remaining elements can exhibit +4, +6 oxidation states as well (thanks to the availability of vacant d orbitals).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






